Advance makes MRI scans more than seven times faster
UC Berkeley physicist David Feinberg, in collaboration with physicians at the University of Minnesota, has combined two new techniques to speed MRI scans of the brain by more than a factor of 10. The faster functional MRI scans will boost the national effort to map the brain’s wiring, called the Human Connectome Project.
January 5, 2011
An international team of physicists and neuroscientists has reported a breakthrough in magnetic resonance imaging that allows brain scans more than seven times faster than currently possible.
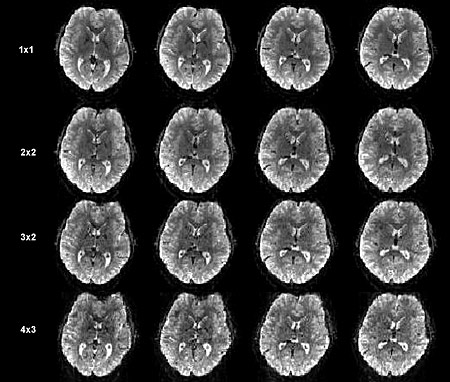
fMRI brain scans without the new acceleration techniques (top row) and with increasing numbers of pulse sequences and slice accelerations. The bottom row was obtained 10 times faster than the top row, although all show similar resolution. Only 4 of the 60 slices of a full, 3-D brain scan are shown. (David Feinberg/UC Berkeley)
In a paper that appeared Dec. 20 in the journal PLoS ONE, a University of California, Berkeley, physicist and colleagues from the University of Minnesota and Oxford University in the United Kingdom describe two improvements that allow full three-dimensional brain scans in less than half a second, instead of the typical 2 to 3 seconds.
“When we made the first images, it was unbelievable how fast we were going,” said first author David Feinberg, a physicist and adjunct professor in UC Berkeley’s Helen Wills Neuroscience Institute and president of the company Advanced MRI Technologies in Sebastopol, Calif. “It was like stepping out of a prop plane into a jet plane. It was that magnitude of difference.”
For neuroscience, in particular, fast scans are critical for capturing the dynamic activity in the brain.
“When a functional MRI study of the brain is performed, about 30 to 60 images covering the entire 3-D brain are repeated hundreds of times like the frames of a movie but, with fMRI, a 3-D movie,” Feinberg said. “By multiplexing the image acquisition for higher speed, a higher frame rate is achieved for more information in a shorter period of time.”
“The brain is a moving target, so the more refined you can sample this activity, the better understanding we will have of the real dynamics of what’s going on here,” added Dr. Marc Raichle, a professor of radiology, neurology, neurobiology, biomedical engineering and psychology at Washington University in St. Louis who has followed Feinberg’s work.
In addition to broadly advancing the field of neural-imaging, the discovery will have an immediate impact on the Human Connectome Project, funded last year by the National Institutes of Health (NIH) to map the connections of the human brain through functional MRI (fMRI) and structural MRI scans of 1,200 healthy adults.
“At the time we submitted our grant proposal for the Human Connectome Project, we had aspirations of acquiring better quality data from our study participants, so this discovery is a tremendous step in helping us accomplish the goals of the project,” said Dr. David Van Essen, a neurobiologist at Washington University and co-leader of the project. “It’s vital that we get the highest quality imaging data possible, so we can infer accurately the brain’s circuitry – how connections are established, and how they perform.”
The faster scans are made possible by combining two technical improvements invented in the past decade that separately boosted scanning speeds two to four times over what was already the fastest MRI technique, echo planar imaging (EPI). Physical limitations of each method prevented further speed improvements, “but together their image accelerations are multiplied,” Feinberg said. The team can now obtain brain scans substantially faster than the time reductions reported in their paper and many times faster than the capabilities of today’s machines.
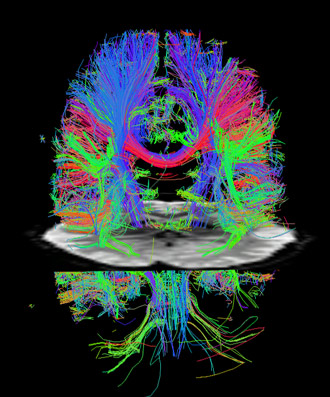
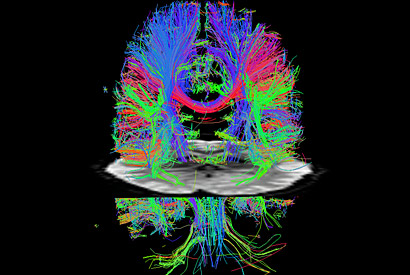
The new techniques accelerate diffusion MRI as well. The colorful tracks show major nerve cell axons in the white matter (cortex) of a resting brain. An fMRI cross sectional image of the brain bisects the diffusion image. (Washington University)
Magnetic resonance imaging works by using a magnetic field and radio waves to probe the environment of hydrogen atoms in water molecules in the body. Because hydrogen atoms in blood, for example, respond differently than atoms in bone or tissue, computers can reconstruct the body’s interior landscape without the use of penetrating X-rays.
Nearly 20 years ago, however, a new type of MRI called functional MRI (fMRI) was developed to highlight areas of the brain using oxygen, and thus presumably engaged in neuronal activity, such as thinking .Using echo planar imaging (EPI), fMRI vividly distinguishes oxygenated blood funneling into working areas of the brain from deoxygenated blood in less active areas.
As with standard MRI, fMRI machines create magnetic fields that vary slightly throughout the brain, providing a different magnetic environment for hydrogen atoms in different areas. The differing magnetic field strengths make the spin of each hydrogen atom precess at different rates, so that when a pulse of radio waves is focused on the head, the atoms respond differently depending on location and on their particular environment. Those that absorb radio energy and then release the energy are detected by magnetic coils surrounding the head, and these signals, or “echoes,” are used to produce an image of the brain.
With EPI, a single pulse of radio waves is used to excite the hydrogen atoms, but the magnetic fields are rapidly reversed several times to elicit about 50 to 100 echoes before the atoms settle down. The multiple echoes provide a high-resolution picture of the brain.
In 2002, Feinberg proposed using a sequence of two radio pulses to obtain two times the information in the same amount of time. Dubbed simultaneous image refocusing (SIR) EPI, it has proved useful in fMRI and for 3-D imaging of neuronal axonal fiber tracks, though the improvement in scanning speed is limited because with a train of more than four times as many echoes, the signal decays and the image resolution drops.
Another acceleration improvement, multiband excitation of several slices using multiple coil detection, was developed in the U.K. at about the same time by David Larkmann for spinal imaging. The technique was recently pioneered for fMRI by Steen Moeller and colleagues at the University of Minnesota. This technique, too, had limitations, primarily because the multiple coils are relatively widely spaced and cannot differentiate very closely spaced images.
In collaboration with Essa Yacoub, senior author on the paper, and Kamil Ugurbil, director of the University of Minnesota’s Center for Magnetic Resonance Research and co-leader of the Human Connectome Project, Feinberg combined these techniques to get significantly greater acceleration than either technique alone while maintaining the same image resolution.
“With the two methods multiplexed, 10, 12 or 16 images the product of their two acceleration factors were read out in one echo train instead of one image,” Feinberg said.
The ability to scan the brain in under 400 milliseconds moves fMRI closer to electroencephalography (EEG) for capturing very rapid sequences of events in the brain.
“Other techniques which capture signals derived from neuronal activity, EEG or MEG, have much higher temporal resolution; hundred microsecond neuronal changes. But MRI has always been very slow, with 2 second temporal resolution,” Feinberg said. “Now MRI is getting down to a few hundred milliseconds to scan the entire brain, and we are beginning to see neuronal network dynamics with the high spatial resolution of MRI.”
The development will impact general fMRI as well as diffusion imaging of axonal fibers in the brain, both of which are needed to achieve the main goal of the Human Connectome Project. Diffusion imaging reveals the axonal fiber networks that are the main nerve connections between areas of the brain, while fMRI shows which areas of the brain are functionally connected, that is, which areas are active together or sequentially during various activities.
“While it simply is not possible to show the billions of synaptic connections in the live human brain, the hope is that understanding patterns of how the normal brain is functionally interacting and structurally connected will lead to insights about diseases that involve miswiring in the brain,” Feinberg said.
“We suspect several neurologic and psychiatric disorders, such as autism and schizophrenia, could be brain connectivity disorders, but we don’t know what normal connectivity is,” Feinberg added. “Although the fMRI and neuronal fiber images do not have the resolution of an electron microscope, the MRI derived Connectome reveals the live human brain and can be combined with genetic and environmental information to identify individual differences in brain circuitry.”
Raichle, a collaborator in the NIH Human Connectome project, is one of the pioneers of “resting state” MRI, in which brain scans are taken of patients not involved in any specific task. He believes that the ongoing spontaneous activity discovered during such scans will tell us about how the brain remains flexible and maintains a degree of homeostatis so that “you know who you are.”
“Being able to sample this ongoing activity at increasing temporal fidelity and precision becomes really important for understanding how the brain is doing this,” Raichle said. “David is superclever at this kind of technical stuff, and I have been cheering him along, saying that the faster we can go, the better we can understand the brain’s spontaneous activity.”
The other authors of the PLoS ONE paper are Steen Moeller and Edward Auerbach of the Center for Magnetic Resonance Research at the University of Minnesota Medical School; Sudhir Ramanna of Advanced MRI Technologies; Matt F. Glasser of Washington University; and Karla L. Miller and Stephen M. Smith of the Oxford Centre for Functional MRI of the Brain at the University of Oxford. Feinberg is also affiliated with the UC San Francisco Department of Radiology.
The work was supported by the NIH’s Human Connectome Project and by other grants from the NIH and from Advanced MRI Technologies.