CRISPR-Cas9 helps uncover genetics of exotic organisms
Genome editing helps reveal how key animal genes set up segmented body plan
December 10, 2015
The simplicity of CRISPR-Cas9 gene editing will soon make studying the genes of any organism, from the simplest slime mold to the octopus, as easy as it now is to study the genes controlling development in standard lab animals such as nematodes, fruit flies, frogs and mice.

Scanning electron microscope images of a normal amphipod (top), about one millimeter long, with color-coded hind jumping legs, and an amphipod that has been genetically modified with CRISPR to grow forward-walking legs instead (bottom). (Arnaud Martin image)
A new study from UC Berkeley illustrates the ease with which CRISPR-Cas9 can knock out genes in exotic animals — in this case, an amphipod or sandhopper — to learn how those genes control growth and development. Researchers wanted to know which genes control the development of appendages on each segment of the amphipod, whose body is like a Swiss army knife with each segment bearing a different blade or tool as an appendage.
In less time than it would have taken two years ago to knock out one gene in the animal, UC Berkeley researchers knocked out six, shedding light on the basic genetic mechanisms that determine leg anatomy in the evolution of animals. By knocking out, one by one, the so-called Hox genes that specify body parts in all animals, they switched the identities of the crustacean’s limbs, transforming a claw into a leg, for example, or a jaw into an antenna.
“For those of us studying non-traditional model organisms in the lab, this has the flavor of a technical revolution,” said UC Berkeley postdoctoral researcher Arnaud Martin. “CRISPR-Cas9 definitely changes the range of possibilities in working with exotic animals, and the amphipod is one of the first.”
The Hox family of genes Martin is investigating is found in all animals, and some biologists argue that an animal can be defined as any creature having Hox genes. They are known to turn on or off a myriad of genes in each segment of the body. Humans’ body segments are most evident in the spine, where each type of spinal vertebra is unique in shape, all under the control of Hox genes.
In the tropical marine amphipod Parhyale hawaiensis, Martin and Patel wanted to know which of the Hox genes function in each of its 19 segments to produce mouthparts at the front, then claws, followed by forward-moving legs, powerful jumping legs that propel the crustacean backwards, swimming legs and finally stumpy “anchor” legs.
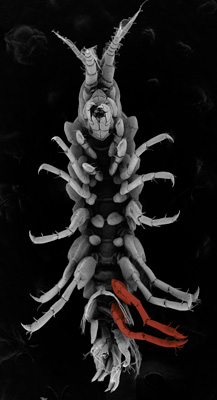
Scanning electron microscope image of a mutant amphipod, with false colors (in red) showing two hind jumping legs that replaced swimming-appendages after CRISPR genome editing. (Arnaud Martin image)
“CRISPR-Cas9 has made a huge impact already in model systems, like the fruit fly Drosophila and the nematode C. elegans, where there are already a lot of tools. People were able to incorporate the technology very quickly and change the way they make mutants in those animals,” said senior author Nipam Patel, a UC Berkeley professor of molecular and cell biology and of integrative biology. “But there are a lot of other animals we want to work with because we can use them to answer really basic evolutionary questions. CRISPR-Cas9 is a great technology for that.”
Other researchers have recently used CRISPR-Cas9 to edit genes in unusual organisms like the sea anemone, the lamprey and butterfly to see whether the tool works, but for the most part these have been “proof-of-principle” experiments using CRISPR-Cas9 to confirm what was already known.
“Here we have gone way beyond just showing that it works in Parhyale and jumped right into answering a really important developmental and evolutionary question: how you set up all of these limbs in the crustacean, and how evolution changes that pattern of limbs by altering Hox gene expression patterns,” Patel said.
Arnaud, Patel and their UC Berkeley colleagues will publish their findings in the Dec. 10 issue of the journal Current Biology.
Master regulatory genes
The Hox complex is a cluster of genes, often referred to as master regulatory genes, that code for protein transcription factors that turn off or on lots of other genes. Starting early in development, they come on in different combinations in different segments of the body, from head to tail. Since they first appeared in our animal ancestors 600 million years ago, evolution has worked with this same complex of genes to produce a huge variety of animal shapes.
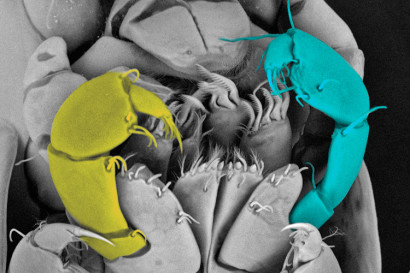
Scanning electron microscope image of the mouthparts of a mutant amphipod hatchling, with color-coded maxillipeds, a recently evolved appendage involved in food processing. The yellow limb is normal, while the blue limb was transformed into a claw using CRISPR. (Arnaud Martin photo)
Amphipods are ideal animals in which to study these genes because each segment has a specific type of leg selected by nine different Hox genes, Martin said.
“They are limbs in the broad sense – really appendages,” he said. “Some have a sensory function – the antennae. Other ones have a prehensile feeding function, like the claws of the lobster. Other appendages have a locomotory function – the legs – or things that are more paddle-shaped for swimming. These differences within an animal are all defined by Hox expression.”
To investigate the function of six of these Hox genes, the researchers employed a hammer approach: use CRISPR-Cas9 enzymes to break and disable one of the six genes in each amphipod embryo, so that the entire organism lacked that one gene alone. They were then able to identify which genes control which appendage.
“In one of the most spectacular transformations, we took the feathery swimming legs located at the front of the abdomen and, by removing one gene, transformed them into what looks exactly like the a large jumping leg normally found on the last thoracic segment,” Patel said. “In another experiment, we basically show exactly how you make an isopod from an amphipod: you move one gene called ‘abdominal-A’ out of the thorax, and this will make all the walking legs similar and pointing all in the same direction.”
Patel noted that in real evolution, genes are not usually broken or eliminated but tweaked, their expression turned on or off in different segments to get different effects. “Without first taking away the gene to see the gross effect, we can’t even begin to guess what the role of just moving around the expression would be,” Patel said. “We can now come up with a code that specifies what kind of limb develops given the combination of Hox genes being expressed there, and we are carrying out new experiments to further test this code.”
Other co-authors of the paper were postdoctoral fellow Julia Serano, graduate students Erin Jarvis and Heather Bruce and undergraduates Jennifer Wang, Shagnik Ray, Carryn Barker and Liam O’Connell. The work was supported by the National Science Foundation (IOS-1257379).
RELATED INFORMATION