Soil prospecting yields wealth of potential antibiotics
Berkeley scientists discover hundreds of antibiotic-like genes by sequencing the genomes of all the microbes in a teaspoon of soil
June 13, 2018
Soil, the source of our best antibiotics, can be more thoroughly mined for new drugs and other useful chemicals with the help of metagenomics, according to UC Berkeley scientists.
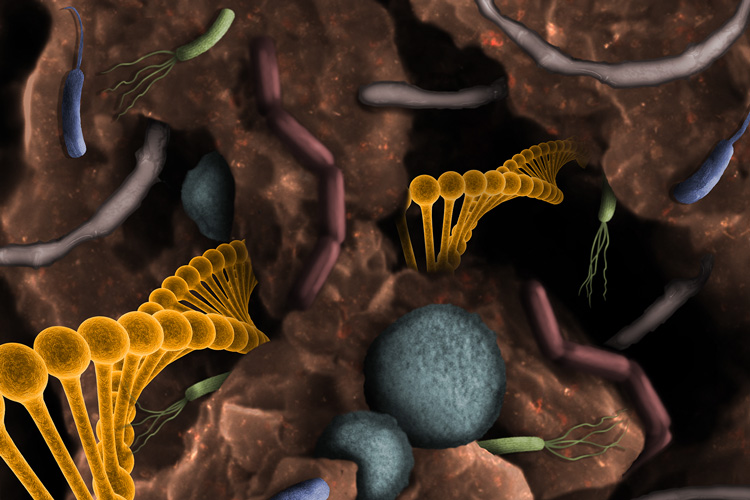
Soil microbes produce many types of chemicals that could potentially be turned into antibiotics, drugs or industrial chemicals. UC Berkeley and Berkeley Lab researchers have found a way to mine this treasure trove by sequencing all the DNA in the thousands of microbes in soil, a technique called metagenomics. Zosia Rostomian image, Berkeley Lab.
In a paper appearing June 13 in the journal Nature, the researchers report sequencing the genomes of every microbe in a teaspoon of soil – so-called metagenomic sequencing – and turning up hundreds of genes for complex and potentially useful molecules that would not have been found otherwise because the microbes cannot be grown in a petri dish.
The genes, many from previously unknown groups of bacteria, likely produce antibiotics or antifungals that the microbes make to defend themselves and which may also be able to combat bacterial or fungal infections in humans.
The search for new antibiotics has become imperative as disease-causing bacteria become increasingly resistant to current drugs while the pipeline of new antibiotics has slowed to a trickle. The Centers for Disease Control and Prevention estimates that each year in the United States, at least 2 million people become infected with bacteria that are resistant to antibiotics and at least 23,000 people die as a direct result of these infections.
From 60 separate 10-gram samples obtained at depths from 4 to 16 inches under a meadow in northern California, the team was able to assemble the genomes of about 1,000 different microbes, both bacteria and Archaea. They are now reporting 360 of them as newly identified bacterial species that are able to produce complex molecules, many of them resembling known antibiotics.
This is the most complex microbial community ever sequenced and assembled through metagenomics, the researchers say. Humus-rich soil is suspected to contain tens of thousands of distinct microbes, most at low numbers. The 1,000 genomes the team was able to assemble varied enormously in abundance: a few genomes each represented about 1 percent of the microbial species in the soil, but most were hundreds to thousands of times more rare.
“Soil is the last frontier from the perspective of genome-resolved metagenomics,” said Jill Banfield, a UC Berkeley professor of earth and planetary science and of environmental science, policy and management and program lead for the microbiology component of the campus’s Innovative Genomics Institute. “It is just full of many, many, many different types of organisms, a lot of them closely related and present in fairly low abundances, so it is hard to tease them apart.“
Antibiotics and antifungals
Though the researchers don’t yet know the exact chemical structures of the hundreds of complex molecules they predict these microbes produce or what these molecules do, they have teamed up with other biologists to find out. They intend to synthesize more than 20 of the newly found genes so that they can be inserted into other organisms where they will be expressed to produce a protein. Then they’ll try to pin down what these proteins do and, if they’re enzymes, what complex molecules they make and whether these molecules have antibiotic or other novel properties.

Meadow from which soil samples were obtained, a climate change study site at the Angelo Coast Range Reserve, part of the University of California’s Natural Reserve System. Jill Banfield photo.
Aside from antibiotic or antifungal activity, these molecules may have functions that can be adapted for the laboratory or industry, much as the CRISPR-Cas9 system was plucked from bacteria to become a revolutionary new gene-editing tool. In the past, soil microbes have been the source of an anticancer drug and an immunosuppressant used to prevent organ rejection.
“Most of these new biosynthetic molecules are coming out of what people know to be the most abundant bacteria in soil, they just hadn’t been found because people didn’t have genomes for them,” Banfield said. “We expect to find novel antibiotics, which could help humanity, but also novel pharmaceuticals more broadly.”
While some researchers in recent years have prospected for antibiotics by extracting DNA from soil and inserting it randomly into bacteria to see what comes out, this “functional metagenomics” can miss molecules made by large clusters of genes, Banfield said.
“Traditionally people have gone to the soil and tried to isolate some of the microbes on a plate of agar, but a very small percentage, less than 1 percent, of the microbes in the soil are able to grow on agar,” said lead author Alexander Crits-Christoph, a UC Berkeley graduate student. “That’s why so many of the antimicrobials we use today come from a couple of families of bacteria. When you assemble these genomes from the environment, you don’t have that selection effect; you get all the stuff that is actually there. This is a targeted approach.”
Meadow microbes
The soil samples were obtained from a meadow at Angelo Coast Range Reserve in Mendocino County, from plots that had been monitored for 15 years as part of a climate change study. Sixty samples were sequenced at the Department of Energy’s Joint Genome Institute and the DNA assembled into genomes by co-author Spencer Diamond, a postdoctoral fellow in the Banfield lab, as part of a DOE study focused on carbon cycling in soil. The researchers estimate that the thousand high-quality genomes represent between 20 and 40 percent of all the microbes in the soil samples.

Members of the UC Berkeley team working beside the South Fork of the Eel River, which runs through the northern California reserve. Jill Banfield photo.
Crits-Christoph scanned the 1,000 nearly complete microbial genomes in search of genes that resemble the genes for other soil-derived antibiotics. Erythromycin is a polyketide, for example, while vancomycin, daptomycin and bacitracin are nonribosomal peptides. He found more than 1,000 polyketide and nonribosomal peptide genes produced by about one-third of the microbes with assembled genomes.
He also looked for potential antibiotic resistance genes near the gene clusters that were identified in the study, a search strategy that works because microbes making antibiotics must first make themselves resistant so as not to commit suicide. And he looked to see when the gene clusters already identified turned on at the same time as genes for microbial competitive behavior and social interactions, because the gene clusters can be important mediators of microbial interactions in soils.
“The chemical ecology of these microbes is really interesting too,” Banfield said. “These are molecules that organisms make to communicate and compete for resources and to acquire resources. Some of them may be quite important to dissolving minerals, for example, to obtain nutrients. They can tell us how these organisms interact with each other.”
The team discovered a large number of biosynthetic genes in novel members of the Acidobacteria, the most abundant bacterial phylum in soil biomes. Two completely new Acidobacterial genomes from different lineages each encoded up to 15 large antibiotic-like gene clusters.
“Two years ago, if you asked people what microbes antibiotics come from, they would say actinomycetes and bacilli, two classes of microbes that really defined microbial antibiotics,” Diamond said. “Now, this study opens an entire new forest, filled with completely new classes of organisms that could conceivably be targets for antibiotic prospecting.”
Other co-authors of the study are Cristina Butterfield and Brian Thomas. The work was funded by the Department of Energy (DOE-SC10010566) and Innovative Genomics Institute.
RELATED INFORMATION