Researchers turn Salmonella into anti-viral gene therapy agent
UC Berkeley researchers have converted Salmonella bacteria from a foodborne pathogen into a safe delivery vehicle for anti-viral agents. They inserted virus-stopping ribozymes into Salmonella that had their ability to cause disease disabled, and then used the bacteria to effectively treat mice infected with cytomegalovirus. It is the first time bacteria have been successfully engineered to treat a viral infection.
February 7, 2011
New experiments at the University of California, Berkeley, may one day lead to anti-viral treatments that involve swallowing Salmonella bacteria, effectively using one bug to stop another.
Researchers at UC Berkeley’s School of Public Health have reprogrammed Salmonella, the same foodborne pathogen that can cause diarrhea, fever and abdominal cramps, into a safe transport vehicle for virus-stopping enzymes. Not only did this technique effectively treat mice infected with cytomegalovirus, it worked as an oral solution that was swallowed instead of injected.
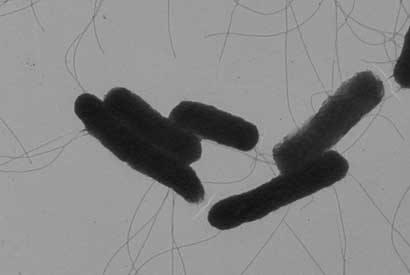
Shown is an image of Salmonella bacteria taken by an electron microscope. Researchers at UC Berkeley have turned this foodborne pathogen into an anti-viral delivery agent. (Sangwei Lu image)
Virologist Fenyong Liu teamed up with bacteriologist Sangwei Lu to develop the innovative technique, which is described in a study to be published online the week of Feb. 7 in the journal Proceedings of the National Academy of Sciences.
“A number of vaccines, including those for polio and smallpox, use live but weakened viruses to build up the immune system. But this is the first time anyone has successfully engineered bacteria for treatment of a viral infection,” said Liu, a UC Berkeley professor at the Division of Infectious Diseases & Vaccinology.
The researchers said Salmonella was particularly appealing because it has evolved to survive the human digestive system, allowing it to be swallowed instead of injected or inhaled.
“This is the first gene therapy treatment for viral infection that can be taken by mouth, which is far more convenient to administer than an injection,” said Lu, a UC Berkeley associate adjunct professor at the Division of Infectious Diseases & Vaccinology. “Moreover, there is already an attenuated strain of Salmonella with a decent track record for safety in humans since it is now used in the vaccine for typhoid (a disease caused by Salmonella Typhi).”
Researchers know that ribozymes, enzymes that are able to target and cut specific RNA molecules, can be used to inactivate a pathogen’s genes. But to do their work, ribozymes need to first get into the cells, and for that they need help.
It so happens that Salmonella is very good at invading cells, so the researchers found a way to use the bacterium as a vector for the RNase P ribozyme that could stop the gene activity of cytomegalovirus, or CMV.
CMV is in the same family of herpes viruses that causes cold sores, mononucleosis and chickenpox. CMV infections are generally mild among healthy individuals, but they can become deadly for people whose immune systems are compromised and are a leading viral cause of mental retardation in newborns.
Previous research by Liu and Lu showed that Salmonella could effectively sneak the anti-viral ribozymes into human cells infected with human cytomegalovirus and reduce the viral load of the cell cultures. This new study put the technique to the test in living mice.
As an added measure of safety, researchers took the attenuated strain of Salmonella and further mutated a gene that the bacteria needs to replicate. They tested the new mutant Salmonella strain in mice and confirmed that the mice did not get sick.
They then cloned the anti-viral ribozymes into a plasmid, or DNA molecules within the bacteria that can replicate. Among mice that had been infected with cytomegalovirus, those that had been given oral doses of the ribozyme-carrying Salmonella survived much better than mice that had not been treated or mice that had been given Salmonella carrying a defective version of the ribozyme. The treated mice lived at least 50 days after infection, whereas the mice in the other two groups died within 25 days after infection.
Moreover, the researchers found that the viral load of mice treated with the ribozyme-carrying Salmonella was 400- to 600-times lower than the viral load for mice given the defective ribozymes and for mice that were untreated.
The researchers pointed out that using bacteria instead of viruses as gene-therapy vectors has a number of advantages.
“Viruses can’t replicate on their own; they must be grown in host cells,” said Lu. “It is more challenging to grow host cells in a lab, and there is always the risk that those cells can be contaminated with unknown viruses. To grow bacteria, you only need to add some bacteria to a simple medium, and the next day you can have 100 billion bacteria ready to go. It’s safer, easier and cheaper as a vector for gene therapy.”
The researchers pointed to the potential for developing this technique into a range of gene-targeting therapeutics. “This study focused on the use of Salmonella and ribozymes to fight infections, but with more research, this method could eventually be used to treat other conditions as well, including cancer,” said Liu.
Other UC Berkeley authors on the paper include lead author Yong Bai and Hao Gong, both post-doctoral researchers in infectious diseases; Hongjian Li, a former post-doctoral researcher in infectious diseases; and Gia-Phong Vu, a graduate student in comparative biochemistry.
The U.S. Department of Agriculture and the National Institutes of Health helped support this research.