Discovery opens door to attacking biofilms that cause chronic infections
Using super-resolution microscopy and continuous fluorescent imaging, UC Berkeley physicists have for the first time revealed the structure of bacterial biofilms, which are responsible for the tenacious nature of bacterial diseases such as cholera and chronic sinusitus. The picture provides new targets for the development of drugs that can tear down these structures.

July 12, 2012
A clever new imaging technique discovered at the University of California, Berkeley, reveals a possible plan of attack for many bacterial diseases, such as cholera, lung infections in cystic fibrosis patients and even chronic sinusitis, that form biofilms that make them resistant to antibiotics.
By devising a new fluorescent labeling strategy and employing super-resolution light microscopy, the researchers were able to examine the structure of sticky plaques called bacterial biofilms that make these infections so tenacious. They also identified genetic targets for potential drugs that could break up the bacterial community and expose the bugs to the killing power of antibiotics.
“Eventually, we want to make these bugs homeless,” said lead researcher Veysel Berk, a postdoctoral fellow in the Department of Physics and the California Institute for Quantitative Biosciences (QB3) at UC Berkeley.
Berk and his co-authors, including Nobel laureate and former UC Berkeley professor Steven Chu, report their findings in the July 13 issue of the journal Science.
“In their natural habitat, 99.9 percent of all bacteria live as a community and attach to surfaces as biofilms; according to the National Institutes of Health, 80 percent of all infections in humans are related to biofilms,” Berk said.
The researchers were able to employ new techniques that allowed them to zoom into a street-level view of these biofilms, where they learned “how they grow from a single cell and come together to form rooms and whole buildings,” Berk said. “Now, we can come up with a logical approach to discovering how to take down their building, or prevent them from forming the building itself.”
Combining super-resolution microscopy with the technique Berk developed, which allows continuous labeling of growing and dividing cells in culture, biologists in many fields will be able to record stop-motion video of “how bacteria build their castles,” he said.
“This work has led to new insights into the development of these complex structures and will no doubt pave the way to new approaches to fighting infectious disease and also bacteriological applications in environmental and industrial settings,” said Chu, a former UC Berkeley professor of physics and of molecular and cell biology and former director of the Lawrence Berkeley National Laboratory.
Bacteria are not loners
The popular view of bacteria is that they are free-living organisms easily kept in check by antibiotics, Berk said. But scientists now realize that bacteria spend most of their lives in colonies or biofilms, even in the human body. While single bacteria may be susceptible to antibiotics, the films can be 1,000 times more resistant and most can only be removed surgically.
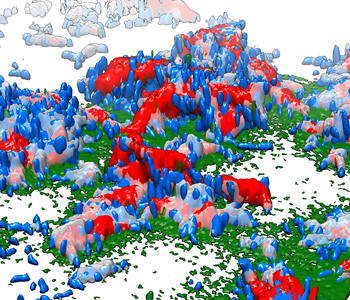
3-D reconstruction of the bacterial biofilm made by cholera bacteria. Bacterial cells (blue) attach to surfaces with a glue-like protein (green) and cement themselves together with another protein (gray). The bacterial clusters then cover themselves with a protective shell (red) made of proteins and sugar molecules. (Veysel Berk image)
Implants, such as pacemakers, stents and artificial joints, occasionally become infected by bacteria that form biofilms. These biofilm sites periodically shed bacteria – adventurers, Berk calls them – which can ignite acute infections and fever. While antibiotics can knock out these free-swimming bacteria and temporally calm down the infection, the biofilm remains untouched. The only permanent solution is removal of the biofilm-coated device and replacement with a new sterilized implant.
A permanent bacterial biofilm in the sinuses can ignite an immune response leading to chronic sinus infections, with symptoms including fever and cold-like symptoms. So far, the most effective treatment is to surgically remove the affected tissue.
Bacteria also form permanent, mostly lifelong, biofilms in the mucus-filled lungs of cystic fibrosis patients and are responsible for the chronic lung infections that lead to early death. Although long-lasting antibiotic treatment helps, it cannot eradicate the infection completely.
To study a biofilm formed by cholera bacteria (Vibrio cholerae), Berk built his own super-resolution microscope in the basement of UC Berkeley’s Stanley Hall based on a 2007 design by coauthor Xiaowei Zhuang, Chu’s former post-doctoral student who is now a professor at Harvard University. To actually see these cells as they divided to form “castles,” Berk devised a new technique called continuous immunostaining that allowed him to track four separate target molecules by means of four separate fluorescent dyes.
He discovered that, over a period of about six hours, a single bacterium laid down a glue to attach itself to a surface, then divided into daughter cells, making certain to cement each daughter to itself before splitting in two. The daughters continued to divide until they formed a cluster – like a brick and mortar building – at which point the bacteria secreted a protein that encased the cluster like the shell of a building.
The clusters are separated by microchannels that may allow nutrients in and waste out, Berk said.
“If we can find a drug to get rid of the glue protein, we can move the building as a whole. Or if we can get rid of the cement protein, we can dissolve everything and collapse the building, providing antibiotic access,” Berk said. “These can be targets for site-specific, antibiotic medicines in the future.”
Super-resolution microscopy: painting with light
Berk is a biologist trained in physics and optics with expertise in imaging the structures of proteins: He was part of a team that a few years ago determined the atomic-scale structures of the ribosome, the cellular machine that translates genetic message into a finished protein.
He suspected that powerful new super-resolution light microscopy could reveal the unknown structure of biofilms. Super-resolution microscopy obtains 10 times better resolution than standard light microscopy – 20 instead of 200 nanometers – by highlighting only part of the image at a time using photo-switchable probes and compiling thousands of images into a single snapshot. The process is much like painting with light – shining a flashlight beam on a dark scene while leaving the camera shutter open. Each snapshot may take a few minutes to compile, but for slow cellular growth, that’s quick enough to obtain a stop-action movie.
The problem was how to label the cells with fluorescent dyes to continuously monitor their growth and division. Normally, biologists attach primary antibodies to cells, then flood the cells with fluorescent dye attached to a secondary antibody that latches onto the primary. They then flush away the excess dye, shine light on the dyed cells and photograph the fluorescence.
Berk suspected that a critically balanced concentration of fluorescent stain – low enough to prevent background, but high enough to have efficient staining – would work just as well and eliminate the need to flush out excess dye for fear it would create a background glow.
“The classical approach is first staining, then destaining, then taking only a single snapshot,” Berk said. “We found a way to do staining and keep all the fluorescent probes inside the solution while we do the imaging, so we can continuously monitor everything, starting from a single cell all the way to a mature biofilm. Instead of one snapshot, we are recording a whole movie.”
“It was a very simple, cool idea, but everyone thought it was crazy,” he said. “Yes, it was crazy, but it worked.”
Berk’s coauthors are Steven Chu, now with the U.S. Department of Energy; Jan Liphardt, UC Berkeley professor of physics and of molecular and cell biology; Xiaowei Zhuang and Graham T. Dempsey of Harvard; Jiunn C. N. Fong and Fitnat H. Yildiz of UC Santa Cruz; and Omer N. Develioglu of Taksim Research Hospital in Istanbul, Turkey.
Related information:
- Molecular Architecture and Assembly Principles of Vibrio cholerae Biofilms (July 13, 2012 Science)