Did bacteria spark evolution of multicellular life?
A new study suggests that bacteria may have helped kick off one of the key events in evolution: the leap from one-celled organisms to many-celled organisms, a development that eventually led to animals, including humans.
October 24, 2012
Bacteria have a bad rap as agents of disease, but scientists are increasingly discovering their many benefits, such as maintaining a healthy gut.
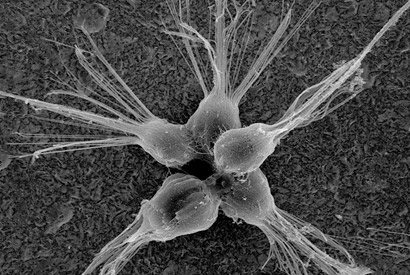
Triggered by the presence of bacteria, the single-celled choanoflagellate Salpingoeca rosetta divides and stays with its sisters to form a colony. One reason may be that the colony is a more efficient way of capturing food, like a “Death Star” sitting amidst the bacteria and chowing down. The colony is about 15 microns in diameter, or less than one-thousandth of an inch across. Scanning electron microscope image courtesy of Nicole King.
A new study now suggests that bacteria may also have helped kick off one of the key events in evolution: the leap from one-celled organisms to many-celled organisms, a development that eventually led to all animals, including humans.
Published this month in the inaugural edition of the new online journal eLife, the study by University of California, Berkeley, and Harvard Medical School scientists involves choanoflagellates (aka “choanos”), the closest living relatives of animals. These microscopic, one-celled organisms sport a long tail or flagellum, tentacles for grabbing food and are members of the ocean’s plankton community. As our closest living relative, choanos offer critical insights into the biology of their last common ancestor with animals, a unicellular or colonial organism that lived and died over 650 million years ago.
“Choanoflagellates evolved not long before the origin of animals and may help reveal how animals first evolved,” said senior author Nicole King, UC Berkeley associate professor of molecular and cell biology.
Since first starting to study choanoflagellates as a post-doc, King has been trying to figure out why some choanoflagellates live their lives as single cells, while others form colonies. After years of dead ends, King and undergraduate researcher Richard Zuzow discovered accidentally that a previously unknown species of bacteria stimulates one choanoflagellate, Salpingoeca rosetta, to form colonies. Because bacteria were abundant in the oceans when animals first evolved, the finding that bacteria influence choano colony formation means it is plausible that bacteria also helped to stimulate multicellularity in the ancestors of animals.
“I would be surprised if bacteria did not influence animal origins, since most animals rely on signals from bacteria for some part of their biology,” King said. “The interaction between bacteria and choanos that we discovered is interesting for evolutionary reasons, for understanding how bacteria interact with other organisms in the oceans, and potentially for discovering mechanisms by which our commensal bacteria are signaling to us.”
No one is sure why choanoflagellates form colonies, said one of the study’s lead authors, UC Berkeley post-doctoral fellow Rosanna Alegado. It may be an effective way of exploiting an abundant food source: instead of individual choanoflagellates rocketing around in search of bacteria to eat, they can form an efficient bacteria-eating “Death Star” that sits in the middle of its food source and chows down.
Whatever the reasons, colonies of unicellular organisms may have led the way to more permanent multicellular conglomerations, and eventually organisms comprised of different cell types specialized for specific functions.
Sequencing the choanoflagellate genome
King’s 12-year search for the trigger of choanoflagellate colony development was reignited in 2005 when she started to prime cultures of the choanoflagellate S. rosetta for a genome sequencing project. The sequencing of another choanoflagellate, the one-celled Monosiga brevicollis, gave some clues into animal origins, but she needed to compare its genome to that of a colony-forming choanoflagellate.
Surprisingly, when Zuzow tried to isolate the colony-forming choanoflagellate by adding antibiotics to the culture dish to kill off residual bacteria, strange things happened, said King.
“When he treated the culture with one cocktail of antibiotics, he saw a bloom of rosette colony formation,” she said, referring to the rose petal-shaped colonies that were floating in the culture media. “When he treated with a different cocktail of antibiotics, that got rid of colony formation altogether.”
That “rather mundane but serendipitous observation” led Zuzow and Alegado to investigate further and discover that only one specific bacterial species in the culture was stimulating colony formation. When other bacteria outnumbered it, or when antibiotics wiped it out, colony formation stopped. Alegado identified the colony-inducing bacteria as the new species, Algoriphagus machipongonensis. While she found that other bacteria in the Algoriphagus genus can also stimulate colony formation, other bacteria like E. coli, common in the human gut, cannot.

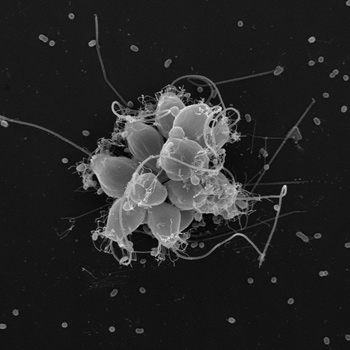
Single choanoflagellates (left) and the rosetta colonies they form in the presence of a newly discovered species of bacteria. Image courtesy of Nicole King.
Working with Jon Clardy of Harvard Medical School, a natural products chemist, the two labs identified a molecule – a fatty acid combined with a lipid that they called RIF-1 – that sits on the surface of bacteria and is the colony development cue produced by the bacteria.
“This molecule may be betraying the presence of bacteria,” Alegado said. “Bacteria just sit around blebbing off little membrane bubbles, and if one of them has this molecule, the choanoflagellates all of a sudden say, ‘Aha, there are some bacteria around here.’”
The signal sets off a predetermined program in the choanoflagellate that leads to cell division and the development of rosettes, she said. The molecule RIF-1 is remarkably potent; choanos detect and respond to it at densities that are about one billionth that of the lowest concentration of sugar that humans can taste in water.
“We are investigating this molecule from many sides. How and why do bacteria make it? How do choanoflagellates respond to it, and why?” King said. She and her team also are analyzing the genome of the colony-forming choanoflagellate and the colony-inducing bacteria for clues to their interaction.
King hopes that this unexpected signaling between choanoflagellates and bacteria can yield insights into other ways in which bacteria influence biology, particularly the biology of the gut.
Coauthors with King, Alegado and Clardy are Zuzow, now a graduate student at Stanford University; Laura Brown, now a faculty member at Indiana University; Shugeng Cao and Renee Dermenjian of Harvard Medical School; and Stephen Fairclough of UC Berkeley. Dermenjian is now at Merck.
RELATED INFORMATION