NIH awards UC Berkeley $7.2 million to advance brain initiative
The National Institutes of Health announced its first research grants through President Barack Obama's BRAIN Initiative, including 3 awards to UC Berkeley totaling nearly $7.2 million over 3 years. The White House also announced a $5.6 million private-public partnership between UC Berkeley and Carl Zeiss Microscopy to improve neural microscopy.

September 30, 2014
The National Institutes of Health today announced its first research grants through President Barack Obama’s BRAIN Initiative, including three awards to the University of California, Berkeley, totaling nearly $7.2 million over three years.
The projects are among 58 funded in this initial wave of NIH grants, involving 100 researchers and a total of $46 million in fiscal year 2014 dollars alone.
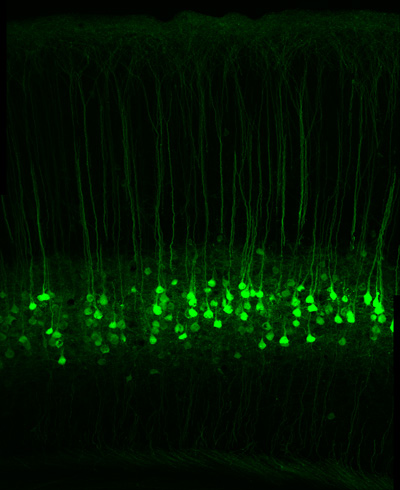
A section of the sensory cortex of a mouse in which cells known as long range projection neurons have been genetically modified to express a green fluorescent protein. John Ngai and colleagues will use single-cell genomics techniques to reveal the diversity of these and other neurons in the brain. (Photo by David Taylor/Hillel Adesnik)
Two of the UC Berkeley grants are from the National Institute of Mental Health (NIMH) to improve imaging tools in order to see greater detail in the brain, and to compile a complete catalog of the thousands of types of neurons in the brain. A third grant is from the National Institute of Neurological Disorders and Stroke (NINDS) to construct probes of neuron signaling that can be switched on or off with light.
“The awards underscore the strength of UC Berkeley’s programs in neuroscience,” said vice chancellor for research Graham Fleming. “Our researchers are leading nation-wide efforts to help us understand how the brain works using new, innovative approaches that draw on insights from many disciplines.”
NIH director Francis Collins, NIMH director Thomas Insel and NINDS director Story Landis made the announcements Tuesday, Sept. 30, during a 10 a.m. press conference at the National Press Club in Washington, D.C.
“The human brain is the most complicated biological structure in the known universe. We’ve only just scratched the surface in understanding how it works — or, unfortunately, doesn’t quite work when disorders and disease occur,” Collins said in a statement. “There’s a big gap between what we want to do in brain research and the technologies available to make exploration possible. These initial awards are part of a 12-year scientific plan focused on developing the tools and technologies needed to make the next leap in understanding the brain. This is just the beginning of an ambitious journey and we’re excited about the possibilities.”
The White House also has scheduled a conference at 3:30 p.m. EDT Tuesday to announce progress since April 2013, when the President first announced his “Grand Challenge” focused on revolutionizing our understanding of the human brain.
At the conference, to be held at the Eisenhower Executive Office Building and streamed live through www.whitehouse.gov/live, John Holdren, assistant to the President for Science and Technology and director of the Office of Science and Technology Policy, will highlight new commitments and investments by the federal government, private sector companies, universities and non-profit organizations in response to the President’s call to action. Paul Alivisatos, UC Berkeley professor of chemistry and director of the Lawrence Berkeley National Laboratory, will moderate a panel discussion following Holdren’s remarks.
Among these is a new $5.6 million public-private collaboration between Carl Zeiss Microscopy and UC Berkeley to support the Berkeley Brain Microscopy Innovation Center (BrainMIC), which will fast-track microscopy development for emerging neurotechnologies and will run an annual course to teach researchers how to use the new technologies. Part of the Helen Wills Neuroscience Institute, the program will generate innovative devices and analytic tools in engineering, computation, chemistry and molecular biology to enable transformative brain science from studies of human cognition to neural circuits in model organisms.
UC Berkeley’s Steven Conolly, professor of electrical engineering and computer sciences, is part of another team that received $1.5 million over three years to develop functional Magnetic Particle Imaging (MPI) to replace functional magnetic resonance imaging (fMRI), which today is the best way to determine the areas of the brain activated during specific tasks. Conolly has built the only functiional MPI imager, which could some day enable direct reading of brain activation without averaging, allowing measurements at the single patient level, which could greatly impact clinical medicine
In May, a UC Berkeley/UC San Francisco collaboration, the Center for Neural Engineering and Prostheses, received a five-year, $26 million BRAIN Initiative grant from the Defense Advanced Research Projects Agency (DARPA) to develop revolutionary and long-lasting treatments for depression, anxiety disorders, addiction and other neuropsychiatric disorders.
The three NIH-funded projects are described below.
Profiling brain cells
John Ngai, the Coates Family Professor of Neuroscience and director of the QB3 Functional Genomics Laboratory, and his UC Berkeley colleagues will receive $4.3 million over three years from NIMH to use new techniques for identifying and isolating different types of neurons, and then sequencing the genes of these cells to discover the full variety of cell types in the brain involved in processes such as memory and learning.
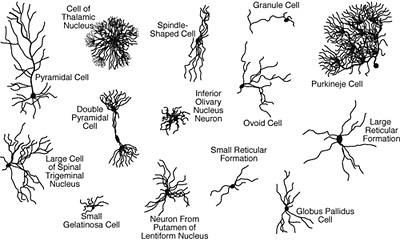
A sampling of the variety of neurons in the brain, as cataloged by Ramon y Cajal in 1899. Scientists still do not know what most of them do.
Surprisingly, Ngai said, neuroscientists do not know how many types of neurons – not to mention the cells that support them – are in the brain. Without such a “parts list,” it will be difficult to understand how individual neurons in a brain circuit ultimately contribute to behavior, in both normal and disease states. But thanks to new techniques that can sequence the active genes in a single cell, his 10-person team will profile all the cells in one part of the brain based on which of the body’s 20,000 genes they express. They will then use this information to catalog the newly-discovered brain cell types.
“Our approach can ultimately be scaled up to generate a complete census of cell types in the brain, a critically needed resource for dissecting nervous system function with modern investigative tools,” said Ngai.
They also plan to use new CRISPR gene editing technology, developed by UC Berkeley professor and team member Jennifer Doudna, to produce genetically engineered mice with each cell type tagged, ready for use by other researchers. Other members of the Berkeley team include neuroscientists Hillel Adesnik, Helen Bateup and Dan Feldman, geneticists Dirk Hockemeyer and Russell Vance, statisticians Sandrine Dudoit and Elizabeth Purdom and computer scientist Nir Yosef.
“This is a watershed moment in terms of the technology available to us today,” Ngai said, noting that 15 years ago he and a colleague had proposed to classify cell types in the brain using single cell techniques, only to be stymied by the technical difficulty at the time. “Knowing how many different types of neurons there are in the brain has been an enormous, unsolved problem since Ramon y Cajal’s initial description of neurons more than 100 years ago. It is exciting that we can now develop and integrate tools from multiple disciplines to reveal the diversity of cell types in the brain, the most complex organ in the body.”
Surface imaging of the brain
David Feinberg, a UC Berkeley adjunct professor of neuroscience, and collaborators at the University of California, San Francisco, Harvard and Duke universities will receive $1.4 million over three years from NIMH to increase the detail or spatial resolution of magnetic resonance imaging (MRI) more than 30 times over today’s most powerful MRI scanners.
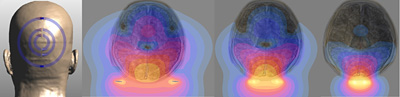
Smaller MRI coils provide much more detail about the surface of the brain, where thinking and learning take place, but less detail about the interior, as illustrated in these simulated vews from the top of the head on the right. David Feinberg image.
“Just as with the Hubble Space Telescope, with clearer and more detailed images you discover new things,” said Feinberg, a physicist who has worked for decades to improve the speed and clarity of MRI imaging. “Assuming we achieve such higher resolution, it will open the door to many new experiments to study brain circuitry.”
Functional MRIs (fMRI) use magnetic fields and radio waves to map areas of the brain that are actively working, allowing neuroscientists or psychologists to pinpoint areas involved in specific tasks, ranging from reading or recognizing faces to emotions such as fear or love. The faint signals from molecules in the brain are typically detected by coils of wire arranged around the head, but these receiver coils need to be large – eight centimeters (3 ¼ inches) in diameter in state-of-the-art machines with 32 coils – in order to record from deep in the brain’s interior.
Functional MRI is sometimes critically referred to as “blobology,” he said, because identifiable regions of brain activity consist of hundreds of thousands of nerve cells, providing only a blobby average of what is happening at any one time.
If you want to study thinking and learning, however, you need focus only on the outer three millimeters of the brain, the grey matter or cortex, Feinberg said.
“The gray matter at the surface of the brain is where the neuron cell bodies and nerve networks lie, so that is where the action is,” he said. “To probe that thin layer, we will use many smaller coils – each with greater sensitivity – to gather more detail in the brain cortex close to the coil.“
The new technique, called MR Corticography (MRCoG) or cortical MRI, would reveal changes in much smaller regions, identifying the cellular layers of the cortex. They hope to see features as small as 200 microns across, or about twice the width of a human hair.
“MR Corticography … synergistically and cleverly combines cutting edge hardware and software technologies that are being pursued by the various experts on this project to allow us to achieve the very challenging goal of creating microscale MR imaging,” said colleague Kawin Setsompop, an assistant professor of radiology at Harvard Medical School and the Athinoula A. Martinos Center for Biomedical Imaging at Massachusetts General Hospital.
“One aim of the project is to develop new technologies to map nerve fibers and brain connectivity,” said Chunlei Liu, assistant professor of radiology at the Brain Imaging and Analysis Center at Duke University School of Medicine.
“This could help bridge the gap between the detail we see now in humans with fMRI and what is going on at the level of the computational networks within the cortex,” Feinberg said.
Pratik Mukherjee, a clinical neuroradiologist and professor of radiology and bioengineering at UCSF and the San Francisco Veterans Administration hospital pointed to many clinical applications of the MRCoG technology, including traumatic brain injury, autism and epilepsy.
“The improved clarity of the cortical imaging promises to improve the diagnosis and surgical evaluation of many forms of epilepsy by better identifying the abnormal cellular architecture in the gray matter that triggers seizures,” Mukherjee said. “This should help the neurosurgeon to resect all of the abnormal region and thereby prevent or at least greatly reduce any further seizures, while sparing nearby normal brain areas that would result in postoperative deficits if removed.”
Photoswitches
Richard Kramer, the CH and Annie Li Chair in Molecular Biology of Diseases and professor of molecular and cell biology, and Ehud Isacoff, director of the Helen Wills Neuroscience Institute and professor of molecular and cell biology, will receive $1.5 million over three years from NINDS to add photoswitches to all the neurotransmitter receptors in the brain so they can be turned on or off with light to study their roles in brain circuits.

Remote control of neurotransmitter receptors with optogenetic pharmacology. Richard Kramer graphic.
Neurons come with a variety of receptors that respond to a variety of neurotransmitters, and figuring out what each of these does is a daunting task.
“Every neurological disease in one way or another involves neurotransmitters; either their synthesis, their uptake or the receptors they act on,” he said. “Parkinson’s disease, Alzheimer’s disease, psychiatric diseases, drugs of abuse – they all act directly or indirectly through neurotransmitters. To understand these diseases at a clinical level, the ability to manipulate the nervous system at the neurotransmitter and receptor level is going to be essential.”
Kramer several years ago devised a way to attach a photoswitch to receptors that respond to an inhibitory transmitter, GABA, so that he can switch them on or off with a pulse of laser light. Isacoff subsequently attached photoswitches to receptors that respond to an excitatory transmitter, glutamate.
The two now propose to add photoswitches to the gamut of receptors that respond to all glutamate and GABA neurotransmitters so that they can tweak them with light to piece together the neural circuits in the brain. This brings optical control to the level of the synapse, where chemical signals transmit information from cell to cell.
“By tagging receptors for optical control, we can selectively manipulate one form at a time and ask, ‘What does it do for a living?'” Kramer said. “Since the flow of information through neural circuits depends on synapses, the next technological step is to bring optogenetic control to the neurotransmitter receptors that underlie synaptic transmission.”
Kramer calls this technique optogenetic pharmacology, because he uses light to understand the role of chemicals in the brain. Focused laser light allows very precise control in the small area of the synapse, as well as rapid on-off switching of very specific biochemical processes.
Kramer and Isacoff plan to use these photoswitched receptors to probe the synapse and the cellular circuits in the brain, and provide the tagged receptors as a tool to researchers around the world.
ADDENDUM 10/28/14: UC Berkeley’s Steven Conolly, professor of bioengineering and of electrical engineering and computer sciences, is part of team led by Massachusetts General Hospital that received $1.6 million over three years from NIH to develop functional Magnetic Particle Imaging (MPI) to replace functional magnetic resonance imaging (fMRI), which today is the best way to determine the areas of the brain activated during specific tasks. Conolly, who operates the Berkeley Imaging Systems Laboratory, has built the only functional MPI imager, which could someday enable direct reading of brain activation without averaging. This would allow brain measurements of single patients rather than averages over many patients, greatly impacting clinical treatment.
RELATED INFORMATION