Do gut microbes shape our evolution?
Biologist proposes that microbiota can steer a host's evolution in new directions
April 20, 2016
Scientists increasingly realize the importance of gut and other microbes to our health and well-being, but one UC Berkeley biologist is asking whether these microbes — our microbiota — might also have played a role in shaping who we are by steering evolution.

The pea aphid, like many organisms, depends on symbiotic bacteria, without which it would die. (Photo by Shipher Wu, National Taiwan University, courtesy of PLOS Biology)
Biologists have gathered evidence that the interdependence between animals and their symbionts — the organisms, typically bacteria, that live in or on them — has consequences for the evolution of both. But Michael Shapira, a UC Berkeley assistant professor of integrative biology, believes that the diverse microbial communities that we harbor have a more profound effect, significantly ratcheting up evolution in an intimate collaboration for survival.
In a paper now online and scheduled for publication in the July edition of the journal Trends in Ecology and Evolution, Shapira, who studies the gut microbes of the nematode C. elegans, reviews evidence that demonstrates how microbiotas affect and contribute to host evolution, either by evolving along with the host, or by stepping in at critical moments to help the host adapt to a new environmental challenge.
These examples, he says, bolster the relatively recent concept of the hologenome, a term referring to the genomes of the host and its microbes together, encompassing perhaps thousands of different types of bacteria on the skin, in the gut and even in reproductive organs. In his recent paper, Shapira elaborates on a 2008 proposal by Tel Aviv University researchers that evolution can act on the hologenome, rather than on the genomes of the host and its microbiota separately. This implies that as the host evolves to suit a changing environment, its microbiota play a critical role in directing and participating in that evolution.
“When I came across the paper by Ilana Zilber-Rosenberg and Eugene Rosenberg describing the hologenome concept, it blew my mind,” Shapira said. “The idea that animals could undergo selection not based solely on their own genome, but with the help of many more, opens the door for previously unimagined evolutionary paths.”
Shapira expands on this idea to encompass some of the newest discoveries about amazing symbiotic relationships between organisms that, because they depend on one another, are tied together for life.
Aphids and their gut bacteria
Examples of co-evolution of hosts and their symbionts are all around us, Shapira said. Aphids have been shown to rely on bacteria to provide essential amino acids the aphids cannot make themselves, and cannot readily obtain from their diet, while the bacteria – a group called Buchnera – get room and, in the form of sap-derived sugars, board.
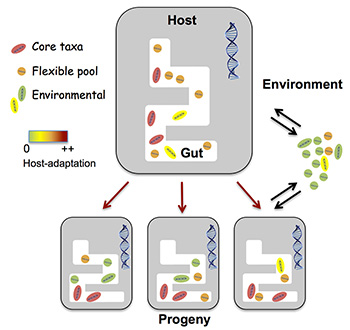
As this schematic illustrates, we all have microbes in our guts: our microbiota. Michael Shapira proposes that not all microbes are the same. Some, core microbes, are important for our existence and evolution, and may even be passed down to our offspring. In addition, a flexible pool of microbes that can be exchanged with similar microbes in the environment is available to assist our survival and adaptation in the face of environmental changes. (Michael Shapira graphic)
Researchers examining the genes of different species of aphids and of their individual gut bacteria found that the emergence of new species of aphids during evolution was mirrored by speciation events in the insects’ Buchnera symbionts. This demonstrates how the linked fates of the two species lead to co-evolution. But Buchnera are not alone. Subsequent work showed that aphids also harbor other symbionts that are less important for, or dependent on, their host, but nevertheless help the insects adapt to new niches in a changing environment.
Together, Shapira suggests, these species represent a basic hologenome, with essential, co-evolving symbionts, but also with a pool of microbes useful for flexibly adapting to a changing environment. Adaptation to new niches can potentially lead to population fragmentation, isolation and, subsequently, to the evolution of a new species. This is a pivotal consequence of life with symbionts, said Shapira, who believes that microbiotas, which involve many types of symbionts, represent an expanded version of this aphid-symbiont relationship.
An experiment performed in fruit flies demonstrates this. When raised on different types of food, flies develop different gut microbiotas, presumably better at handling the available food.
The surprising outcome, however, was that “within one generation, the flies developed mate preference for their own group, ignoring the others, and that this was dependent on the microbes in the gut that helped them utilize the food,” he said. “This led to de facto reproductive isolation of two populations and could facilitate future speciation, that is, real reproductive isolation – a genetic barrier preventing members of the two groups from parenting viable or fertile progeny.”
Nematode’s upset tummies
In his own lab, he is raising nematodes on soil enriched with different types of produce – sugary versus fibrous, for example – and finds that no matter what the food source and the resulting environmental microbial diversity, worms have similar sets of bacteria in their guts. Some 32 types, in fact. The balance shifts with different food, as has been seen in humans as well. People with diets high in fat, for example, have a different microbiota than do vegans, though in humans it is far more difficult to identify a core microbiota.
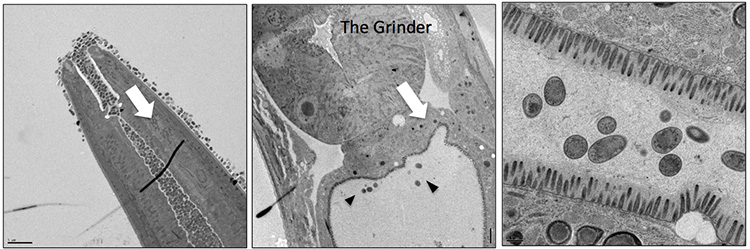
The millimeter-long nematode C. elegans (left) gulps bacteria and grinds it up for food (center). But some bacteria live permanently in its gut (center, black arrows). A few of these enterobacteria, each about 1 micron across, can be seen at high magnification in the nematode’s gut (right). (Electron microscope images by Maureen Berg, UC Berkeley)
Studies have shown that these gut bacteria contribute to many aspects of the host’s life, including development, fertility, metabolism, immunity and behavior. Microbe-free mice don’t develop a robust immune system, for example, while nematodes rely on some gut microbes to fight off bad bugs.
In his paper, Shapira proposes that animals and a set of core bacteria – or in general, a host with its core microbiota – evolve together, adapting as they can to changing conditions and perhaps, over time, becoming new species. The other bacteria swimming around in our guts are less tied to our essential functions, but are influenced by the changing environment, and ideally serve as a resource to help us adapt to sudden changes in diet or toxins, for example.
In thinking about these possibilities, Shapira draws from various examples of symbiosis. One recent experiment showed, for example, that an insect, the broad-headed stink bug, was able to survive pesticide exposure thanks to acquisition of one type of gut microbe that detoxified the pesticide – a boon for the bug but a service needed from the microbe only in one unique situation.
“In my mind, this is an example of the advantages of having a flexible pool of microbes. You can exchange them with the environment, you can get the strains that are better able to protect yourself,” he said. “That’s one way to achieve adaptation.”
Shapira’s proposal implies, too, that some parts of the microbiota — that is, the core microbiota — can likely be passed on to an individual’s children, but that other parts, belonging to the flexible pool, could be exchanged with the environment. Changes in either the host or the microbes change the entire hologenome.
“With the growing understanding that all animals are in fact in a symbiotic relationship with complex microbial communities, the framework to consider how symbiotic interactions shape host evolution should be expanded,” he said.
Shapira plans future experiments with C. elegans to test these ideas and clarify the mutually beneficial relationship between hosts and their microbiotas.
RELATED INFORMATION