Can some types of fat protect us from brain disease?
A newly discovered stress response pathway relies on fat molecules to mediate cellular health

September 8, 2016
An intriguing finding in nematode worms suggests that having a little bit of extra fat may help reduce the risk of developing some neurodegenerative diseases, such as Huntington’s, Parkinson’s and Alzheimer’s diseases.

Against a background of nematode worms, this graphic depicts the stressed mitochondria (lower right, blue and red) accumulating lipid droplets (red balls) in the interior or cytosol of the cell. The molecular structure of the two key lipid components in the newly discovered signaling pathway are ceramide (right) and cardiolipin (upper left). The background image shows a picture of the nematode C. elegans with clumps of the Huntington’s aggregates (bright green because they’re tagged with green fluorescent protein) in the body wall muscle cells. (Hyun-eui Kim and Sarah Uhlein Tronnes image)
What these illnesses have in common is that they’re caused by abnormal proteins that accummulate in or between brain cells to form plaques, producing damage that causes mental decline and early death.
Huntington’s disease, for example, is caused by aggregating proteins inside brain neurons that ultimately lead to motor dysfunction, personality changes, depression and dementia, usually progressing rapidly after onset in people’s 40s.
These protein aggregates – called Huntington’s aggregates – have been linked to problems with the repair system that nerve cells rely on to fix proteins that fold incorrectly: the cell’s so-called protein folding response. Misfolded proteins can make other proteins fold incorrectly, creating a chain reaction of misfolded proteins that form clumps that the cell can’t deal with.
When University of California, Berkeley, researchers perturbed the powerhouses of the cell, the mitochondria, in a strain of the nematode C. elegans that mimics Huntington’s disease, they saw their worms grow fat. They traced the effect to increased production of a specific type of lipid that, surprisingly, prevented the formation of aggregate proteins. The fat, they found, was required to turn on genes that protected the animals and cells from Huntington’s disease, revealing a new pathway that could be harnessed to treat the disease.
The same proved true in human cell lines cultured in a dish.
“We found that the worms and human cells were almost completely protected from the Huntington’s aggregates when we turned on this response,” said Andrew Dillin, the Thomas and Stacey Siebel Distinguished Chair in Stem Cell Research in UC Berkeley’s Department of Molecular and Cell Biology and a Howard Hughes Medical Institute investigator.
They subsequently treated worms and human cells with Huntington’s disease with drugs that prevented the cell from sweeping up and storing the lipid, called ceramide, and saw the same protective effect.
“If we could manipulate this lipid pathway, we could go after Huntington’s disease, because in our studies the drugs were really beneficial,” he said. “This is poised to take to the next level.”
Dillin has already begun experiments in mice with Huntington’s disease to see if the drugs result in a better outcome. He published his latest findings online Sept. 8 in the journal Cell.
How Huntington’s disease causes wasting
In an accompanying paper in the same issue of Cell, Dillin also reports that stressing neurons in the brain makes them release a hormone, serotonin, that sends alert messages throughout the body that the brain cells are under attack, setting off a similar stress response in cells far from the brain. In diseases like Huntington’s, mental decline is also associated with peripheral metabolic defects and muscle decline.
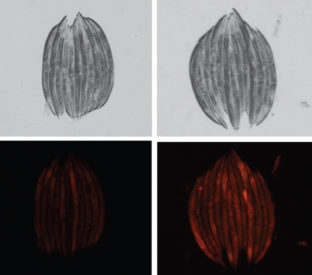
When the researchers knocked out a part of the nematode’s mitochondrial protein folding network (right), the worms grew fatter than normal worms (left, stacked together) and accumulated more fat, as shown by the increased red staining. (Hyun-eui Kim image)
“The serotonin release dramatically changes the metabolic output of peripheral cells and the sources they use for fuel, so we think it is instituting a large-scale metabolic rewiring, maybe to protect the neurons in the brain,” he said. “If you begin to shut down the periphery and stop using the limited resources it utilizes, then more of those resources can be shifted to brain metabolic activity. This might be a very clever way to try to save the brain by having the body waste away.”
While Dillin discovered the ability of mitochondria to communicate between different cells and tissues several years ago, the new study pinpoints serotonin as a primary driver of this metabolic response, he said.
Dillin noted that drugs that lower levels of serotonin have long been used to treat depression and other psychiatric manifestations of neurodegenerative diseases, but the new findings suggest these medications may have more widespread use in age-related disease than was previously thought. These findings have broad implications not only for the potential treatment of neurodegenerative disorders, but for further understanding the impact of neurological disease on metabolism and stress responses throughout the body.
Mitochondria key to brain degeneration
Both discoveries came from studies of mitochondria, the powerhouses of the cell that burn nutrients for energy but also play a key role in signaling, cell death and growth. Over the past several years, increasing evidence has associated mitochondrial dysfunctions with aging and age-onset protein misfolding diseases such as Alzheimer’s, Parkinson’s and Huntington’s.
Dillin is particularly interested in Huntington’s disease, which is inherited and strikes people in their 40s and 50s, inevitably leading to a wasting death. The genetic cause is well-known – expansion of a part of a gene that produces a protein with too many added glutamine amino acids. How this glutamine-rich protein leads to symptoms is only graduatlly being revealed.
While investigating mitochondria in nematodes genetically engineered to have Huntington’s disease, Dillin and his colleagues discovered that the abnormal proteins actually aggregate on the mitochondria, and that this ramps up the protein folding response within the cell, flooding both the mitochrondria and the cell interior with nearly 100 types of so-called heat shock proteins to try to fix the misfolded proteins. The heat-shock proteins act as mitochondrial chaperones to assist in the import and folding of mitochondrial proteins synthesized outside of mitochondria.
The researchers were surprised to find that knockdown of one specific mitochondrial chaperone, mtHSP70, elicited a unique stress response mediated by fat accumulation, resulting in improved protein folding in the interior or cytosol of the cell. Drugs that activate this novel stress response pathway, which they call the mitochondrial-to-cytosolic stress response, protected both nematodes and cultured human cells with Huntington´s disease from protein-folding damage.
“Maybe there is a way to use one drug to alter the mitochondrial signal and another drug to alter the communciation signal from the brain,” he said. “You would never see these two effects if you were studying protein folding in a tissue culture dish, because you don’t have the whole organism, C. elegans, in which you can look at the signals being communicated.”
Co-authors of the fat study include Hyun-Eui Kim, Ana Rodrigues Grant, Milos Simic, Rebecca Kohnz, Daniel Nomura, Jenni Durieux, Celine Riera, Melissa Sanchez, Erik Kapernick and Suzanne Wolff at UC Berkeley. The second study was co-authored by Kristen Berendzen, Jenni Durieux, Ye Tian, Hyun-eui Kim and Suzanne Wolff of UC Berkeley, in collaboration with Li-Wa Shao and Ying Liu of Peking University in Beijing.
The studies are supported by the Howard Hughes Medical Institute, National Institutes of Health, Glenn Foundation for Medical Research, and Jane Coffin Childs Memorial Fund for Medical Research.
RELATED INFORMATION