Researchers find out why some stress is good for you
Chronic stress is known to cause major health problems, yet acute stress is thought to improve people's performance and health. A new study by UC Berkeley professor Daniela Kaufer shows why that is. Stress generates new nerve cells in the brain that, two weeks later, help people learn better.

April 16, 2013
Overworked and stressed out? Look on the bright side. Some stress is good for you.
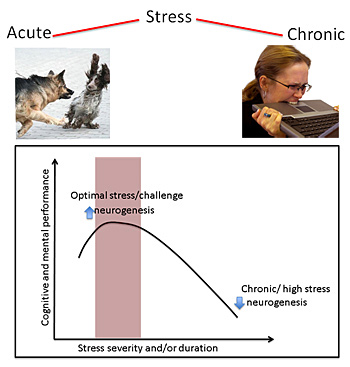
While too little stress can lead to boredom and depression, too much can cause anxiety and poor health. The right amount of acute stress, however, tunes up the brain and improves performance and health. iStock images.
“You always think about stress as a really bad thing, but it’s not,” said Daniela Kaufer, associate professor of integrative biology at the University of California, Berkeley. “Some amounts of stress are good to push you just to the level of optimal alertness, behavioral and cognitive performance.”
New research by Kaufer and UC Berkeley post-doctoral fellow Elizabeth Kirby has uncovered exactly how acute stress – short-lived, not chronic – primes the brain for improved performance.
In studies on rats, they found that significant, but brief stressful events caused stem cells in their brains to proliferate into new nerve cells that, when mature two weeks later, improved the rats’ mental performance.
“I think intermittent stressful events are probably what keeps the brain more alert, and you perform better when you are alert,” she said.
Kaufer, Kirby and their colleagues in UC Berkeley’s Helen Wills Neuroscience Institute describe their results in a paper published April 16 in the new open access online journal eLife.
The UC Berkeley researchers’ findings, “in general, reinforce the notion that stress hormones help an animal adapt – after all, remembering the place where something stressful happened is beneficial to deal with future situations in the same place,” said Bruce McEwen, head of the Harold and Margaret Milliken Hatch Laboratory of Neuroendocrinology at The Rockefeller University, who was not involved in the study.
Kaufer is especially interested in how both acute and chronic stress affect memory, and since the brain’s hippocampus is critical to memory, she and her colleagues focused on the effects of stress on neural stem cells in the hippocampus of the adult rat brain. Neural stem cells are a sort of generic or progenitor brain cell that, depending on chemical triggers, can mature into neurons, astrocytes or other cells in the brain. The dentate gyrus of the hippocampus is one of only two areas in the brain that generate new brain cells in adults, and is highly sensitive to glucocorticoid stress hormones, Kaufer said.
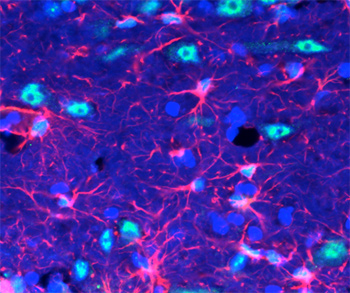
Brain cells called astrocytes (pink) appear to be key players in the response to acute stress. Stress hormones stimulate astrocytes to release fibroblast growth factor 2 (green), which in turn lead to new neurons (blue). Image by Daniela Kaufer & Liz Kirby.
Much research has demonstrated that chronic stress elevates levels of glucocorticoid stress hormones, which suppresses the production of new neurons in the hippocampus, impairing memory. This is in addition to the effect that chronically elevated levels of stress hormones have on the entire body, such as increasing the risk of chronic obesity, heart disease and depression.
Less is known about the effects of acute stress, Kaufer said, and studies have been conflicting.
To clear up the confusion, Kirby subjected rats to what, to them, is acute but short-lived stress – immobilization in their cages for a few hours. This led to stress hormone (corticosterone) levels as high as those from chronic stress, though for only a few hours. The stress doubled the proliferation of new brain cells in the hippocampus, specifically in the dorsal dentate gyrus.
Kirby discovered that the stressed rats performed better on a memory test two weeks after the stressful event, but not two days after the event. Using special cell labeling techniques, the researchers established that the new nerve cells triggered by the acute stress were the same ones involved in learning new tasks two weeks later.
“In terms of survival, the nerve cell proliferation doesn’t help you immediately after the stress, because it takes time for the cells to become mature, functioning neurons,” Kaufer said. “But in the natural environment, where acute stress happens on a regular basis, it will keep the animal more alert, more attuned to the environment and to what actually is a threat or not a threat.”
They also found that nerve cell proliferation after acute stress was triggered by the release of a protein, fibroblast growth factor 2 (FGF2), by astrocytes — brain cells formerly thought of as support cells, but that now appear to play a more critical role in regulating neurons.
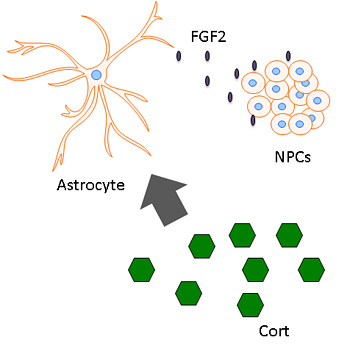
Corticosterone (green hexagons), a glucocorticoid hormone related to stress, stimulates astrocytes to release FGF2, which triggers the generation of new neurons from neural stem cells.
“The FGF2 involvement is interesting, because FGF2 deficiency is associated with depressive-like behaviors in animals and is linked to depression in humans,” McEwen said.
Kaufer noted that exposure to acute, intense stress can sometimes be harmful, leading, for example, to post-traumatic stress disorder. Further research could help to identify the factors that determine whether a response to stress is good or bad.
“I think the ultimate message is an optimistic one,” she concluded. “Stress can be something that makes you better, but it is a question of how much, how long and how you interpret or perceive it.”
The eLife paper was coauthored by UC Berkeley colleagues Sandra E Muroy, Wayne G. Sun and David Covarrubias of the Department of Molecular and Cell Biology; Megan J. Leong of the Helen Wills Neuroscience Institute; and Laurel A. Barchas of the Department of Integrative Biology. Kirby is now a post-doctoral fellow at Stanford University.
Kaufer’s research was funded by a BRAINS (Biobehavioral Research Awards for Innovative New Scientists) award from the National Institute of Mental Health of the National Institutes of Health (R01 MH087495) and the National Alliance for Research on Schizophrenia and Depression. Kirby was supported by fellowships from the California Institute for Regenerative Medicine and the U.S. Department of Defense.
RELATED INFORMATION